HeCNOS AWARD Winners Featured at Expo 2025: Healthcare Startups to Watch (Part 1)

With the Osaka-Kansai Expo 2025 well underway, innovation is taking center stage at the Reborn Challenge , located inside the Osaka Healthcare Pavilion. This unique program highlights the strengths and creativity of over 400 Japan-based SMEs and startups , featuring new technologies and breakthroughs under 26 weekly themes through interactive exhibits and experiences.
As part of these efforts, the Osaka Business Development Agency (OBDA) launched the HeCNOS AWARD to recognize the most promising startups in healthcare and carbon neutrality. Award-winning companies earned the opportunity to showcase their cutting-edge technologies at Expo 2025, inside the Reborn Challenge pavilion .
Recently, we hosted two dynamic programs:
- ・Smart Healthcare Town: a hub for startups redefining the future of healthcare
- ・Carbon Neutral Treasure Hunt: showcasing technologies shaping a sustainable society
Over the next four newsletters, we’ll feature some of the HeCNOS AWARD-winning startups shown at Expo 2025. Today, we’re introducing three healthcare innovators, and letting them explain their startups in their own words.
Rebirthel

Q: What problem does Rebirthel solve?
A: Infectious diseases continue to pose a global threat, particularly with the emergence of new viruses like COVID-19. Current treatments, primarily antibody-based, are limited in their effectiveness against unknown viruses. We aim to fill this critical gap.
Q: What technology are you developing?
A: We use iPS cells to produce and train killer T-cells—immune “special forces” that attack virus-infected cells—to deliver novel therapies to patients. This enables defense even against unknown viruses.
Q: What sets you apart?
A: Unlike companies focused on antibodies or donor cells, we create highly potent killer T-cells from iPS cells. It is technically challenging, and thus protected by patents, creating a high entry barrier.
Q: What’s your vision and key milestones?
A: Our goal is to start Phase I clinical trials within three years and deliver rapid-response therapies in 5–10 years, aligned with the G7’s “100-day Mission.” Scaling GMP manufacturing is our biggest challenge, so we seek pharma partners and strategic investors to accelerate commercialization and global expansion.

Masunori Kajikawa, President and CEO of Rebirthel
TearExo

Ryo Horikawa demonstrating the testing method
Q: What problem does TearExo solve?
A: Breast cancer is one of the leading causes of death worldwide, and in Asia, screening access and diagnostic accuracy remain big challenges. Mammography often struggles with dense breast tissues, making early detection difficult.
Q: What technology is TearExo developing?
A: We’re creating a non-invasive cancer test that uses tears to detect breast cancer. Tears carry biomarkers similar to blood, including cancer-derived exosomes—tiny vesicles that change when cancer develops. Detecting these enables early diagnosis.
Q: How does it work and what’s the benefit?
A: Collection is simple—just a small strip at the eye corner. No pain, no hospital visit. In the future, we aim to make screening possible in pharmacies, clinics, even beauty salons, enabling frequent and accessible testing.
Q: How early can it detect cancer?
A: As early as Stage 1, as confirmed by university studies comparing tears before and after tumor removal surgery.
Q: What’s your IP status and global strategy?
A: We hold 7 patents in Japan and key patents in the U.S., Europe, and China. We’re targeting the U.S. and Europe for private healthcare markets and Asia for its screening challenges. Interest from overseas regulators is already strong.
Q: Key milestones?
A: Each analysis currently takes about 5 minutes per sample. Our near-term goal is 1-week turnaround for postal samples and, ultimately, real-time results in 10–15 minutes at point-of-care settings.
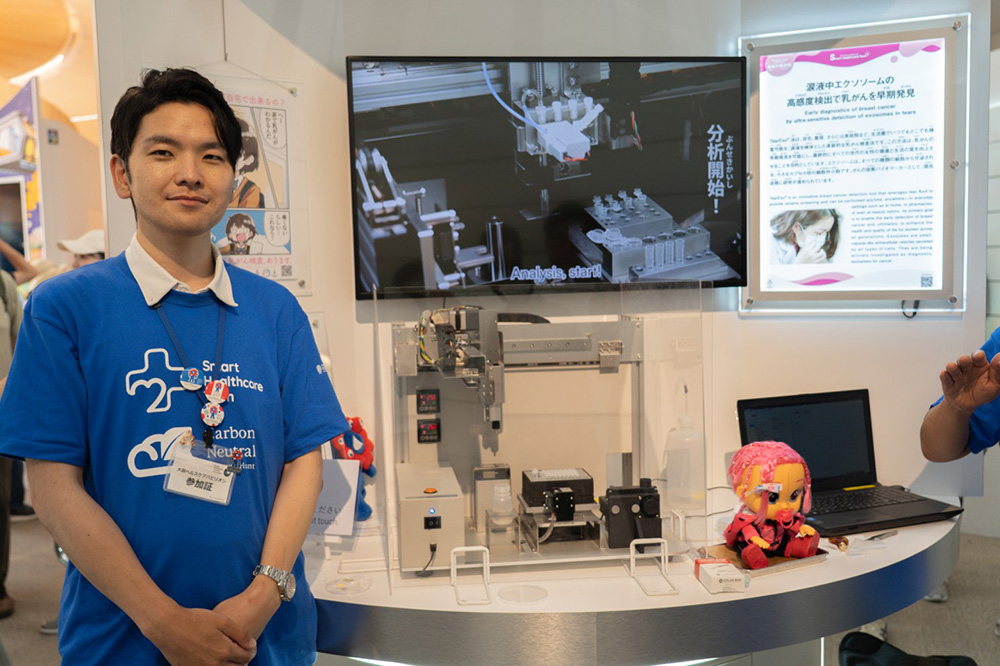
Ryo Horikawa, CEO of TearExo
Fcuro
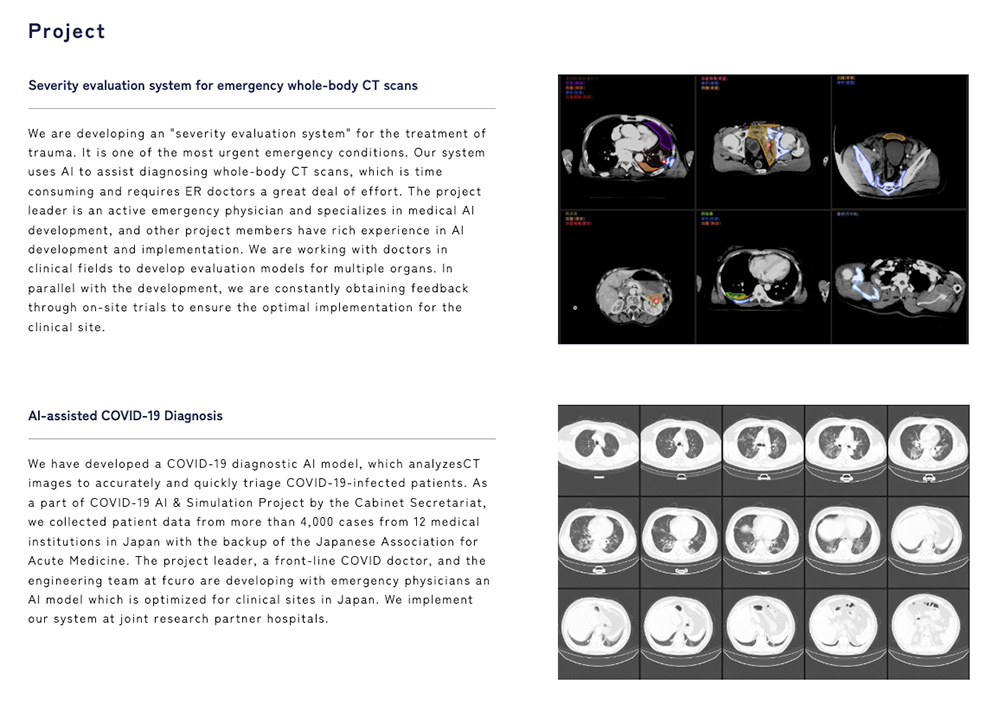
Image: screenshot from official website
Q: What problem does Fcuro solve in emergency medicine?
A: In a trauma emergency, doctors have less than 15 minutes to start treatment, but resuscitation and total-body CT scanning take about 10 minutes—leaving only 5 minutes for diagnosis. Visually inspecting over 1,000 CT images is too slow, leading to delays or missed injuries.
Q: How does your AI solution work?
A: Our system analyzes total-body CT scans and highlights abnormalities in about 10 seconds, guiding doctors to critical injuries instantly. This dramatically improves speed and accuracy of diagnosis in life-or-death situations.
Q: What makes Fcuro different?
A: Unlike most AI tools focused on single organs or specific diseases, we specialize in emergency medicine and whole-body anomaly detection. Our technology also serves as a foundation for building advanced diagnostic AI in the future.
Q: What’s your progress and IP status?
A: We’ve filed about 10 patents covering our algorithm and system integration, both in Japan and internationally.
Q: What are your next milestones?
A: We’ve started real-world implementation at Emergency Hospitals, aim for medical device approval within a few years, and nationwide rollout within four years.
Q: How about global expansion?
A: We’re already collaborating with hospitals in the U.S. and are seeking partners in Asia for joint research and market entry. We’re prioritizing Asia, where traffic accidents are frequent and CT access is limited.

Shun Honda, Engineer at fcuro
About the Event
Enjoy happy & healthy livingSmart Healthcare Town
Exhibition period at the Osaka-Kansai Expo: June 24th Tue –June 30th Mon, 2025
Article Source
Osaka Startup Digest
Exploring the startup ecosystem of Osaka & Kansai.